Designed with You in Mind
The first thing you need to know is our patient registry is a research study. The goal of this study will be to help researchers, clinicians or industry partners learn about MdDS. This article will help you understand how patient data is used and benefits to participation.
Designed to Be Easy
Living with MdDS can make complex tasks feel overwhelming. That’s why the registry is designed to be quick, accessible and flexible. Just go with the flow!
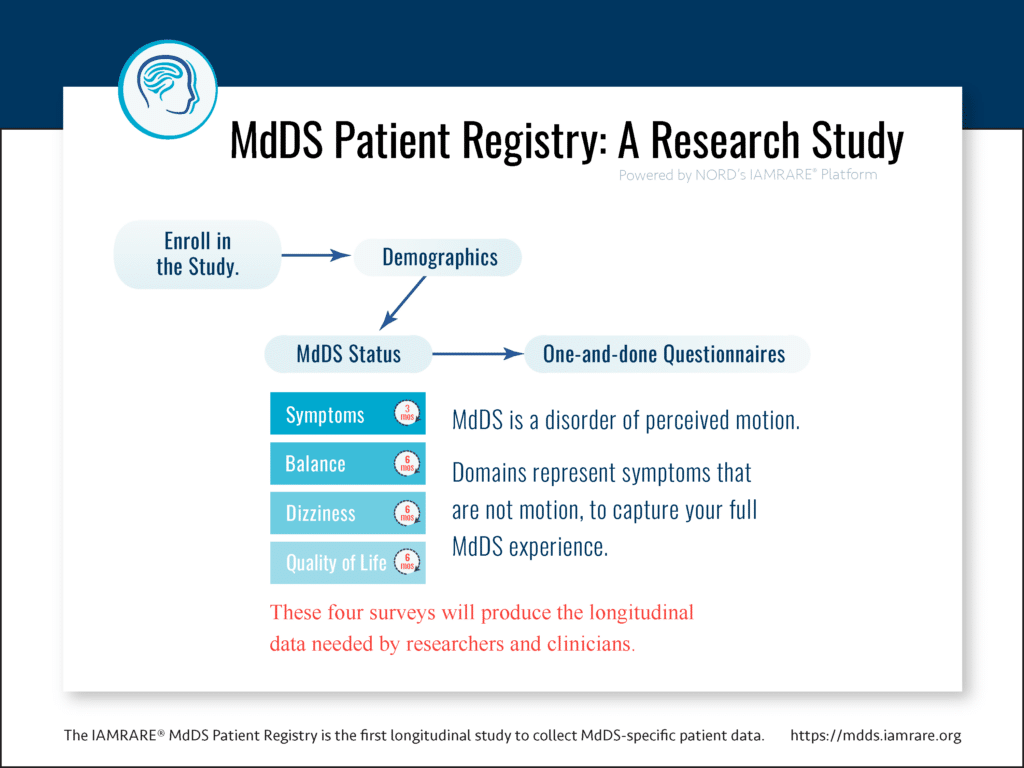
MdDS Status Surveys
The Symptoms Survey is the gateway to gathering the data that can help researchers explore potential treatments or launch clinical trials for MdDS. Combined with the Balance, Dizziness, and Quality of Life surveys, and supplemental questionnaires, we aim to capture the whole of your MdDS experience.
Developed with the guidance of two advisory boards, the surveys and questionnaires were thoughtfully designed to be easy. And in an historical first, the surveys are the first to collect MdDS-specific patient data longitudinally.
What does “longitudinal” mean?
Longitudinal data is what clinicians and researchers alike are asking for! Individual patient data, collected repeatedly over time, illustrates the progression of that individual patient’s condition. This progression is also known as its natural history. Your data in combination with data collected from others in the MdDS community can offer a deeper understanding of MdDS, uncover patterns, and improve treatments.
It’s important to retake the surveys periodically.
We promise you, they’re easy! The registry will even remind you when it’s time to retake a survey, or to complete one if you’ve saved it to finish later. Participants who’ve completed surveys will have the opportunity to be notified about clinical trials, among other benefits.
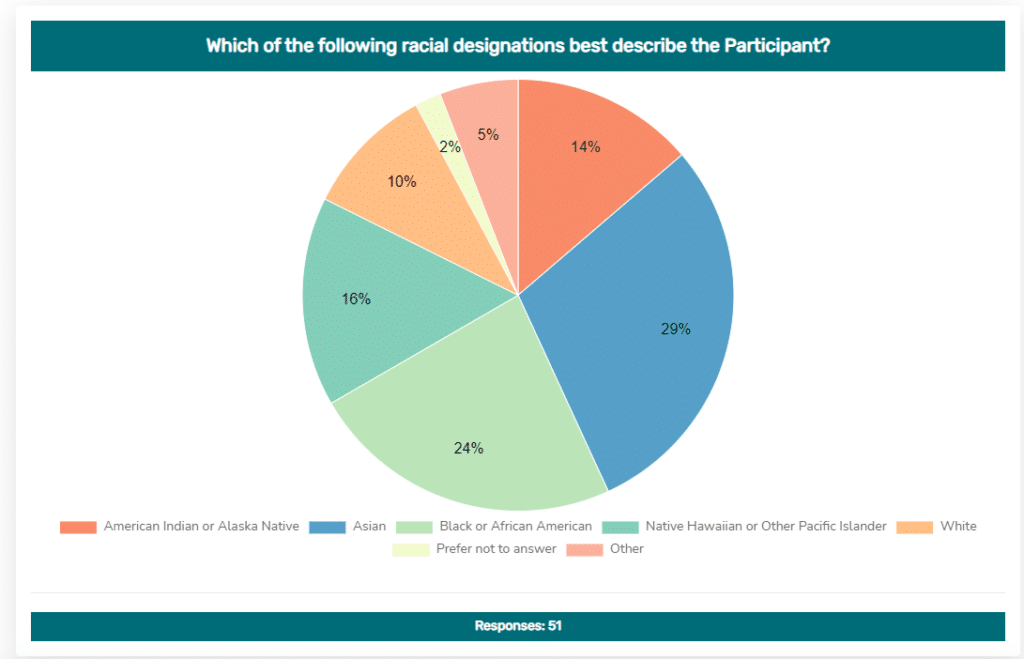
Other Benefits of Participation
Ever wonder how many others are like you? After submitting a survey, you’ll be able to see graphs of aggregate data for the questions you’ve answered. An example using a race question is shown above. The graph shows data from all participants who answered the same question about race. The data is de-identified to protect each participant’s privacy. This type of aggregate, de-identified patient data will be analyzed to inform healthcare providers of information that can improve health care standards and your experience at point-of-care.
We hope this article answers your questions about the MdDS Patient Registry study design, ease of participation, and its impact. Participating in the MdDS Patient Registry is truly one of the most impactful and consequential contributions you can make in working towards the development of treatments and cures.
The call-to-action block may be LEARN MORE or JOIN TODAY depending on how and when this article is published.
Read the related posts and learn more about the MdDS Patient Registry!